CORIg™: Continuous Centrifuge
Uniting forces to provide exceptional separation. True continuous separation with low shear.

Why CORIg?
CORIg™ is the first single-use centrifuge with true continuous discharge and gentle cell handling. Its novel rotational liquid management technology enables runs of over 30 days and delivers a 4-8 fold improvement in throughput within a small footprint.
Key Features
- High Recovery Without Shear: Typically achieves 95-99% cell recovery with unchanged viability.
- Unmatched Versatility: Handles cultures from 0.1 million to >350 million cells/mL.
- Smart: Fully automated with non-invasive turbidity and pressure sensors.
Automated Applications
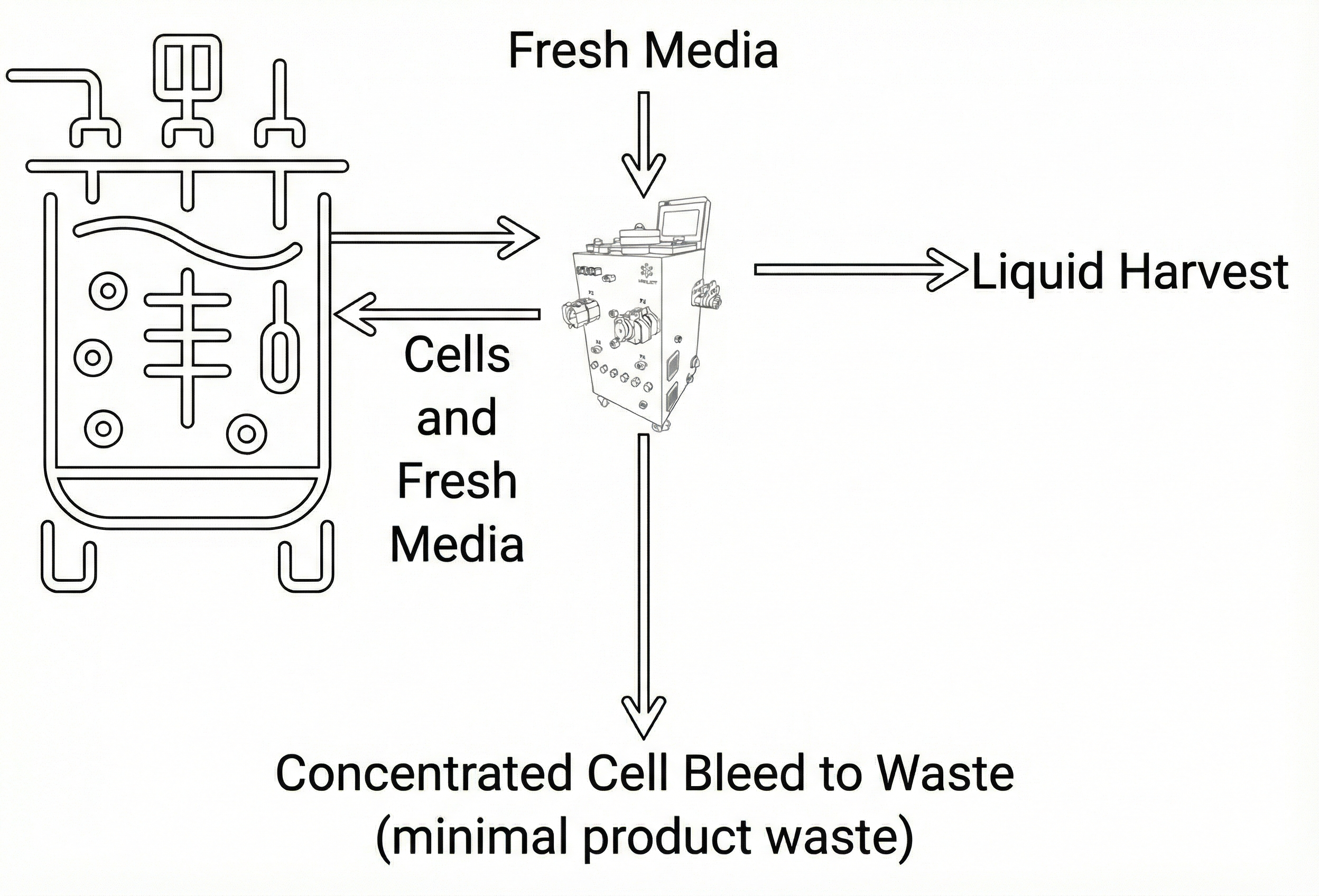
Figure: CORIg Perfusion Workflow
- Perfusion (P): Intelligent cell bleed minimizes liquid product loss.
- Liquid Harvest (L): Efficiently recovers maximum product in liquid.
- Solids/Cells Concentration and Washing (S-B): Concentration and washing of cells.
CORIg-midi Technical Specifications
| Functional Specifications | |
|---|---|
| Max Centrifugal Force | 2000g |
| Typical Process Flow Rate | 80-150 L/hr |
| Inlet Cell Density | 0.05-350 X 106 cells/mL |
| Max Outlet Cell Density | 400 X 106 cells/mL |
| Physical & Utility Specs | |
|---|---|
| Floor Space | 54.5 cm X 50.0 cm (2.93 sq ft) |
| Height (Closed) | 122.5 cm |
| Supply Voltage | 100-250 VAC |
| Instrument Air | >60 psi |




